Structure of erythrose-4-phosphate dehydrogenase from Acinetobacter baumannii at 3.00 A resolution
Viswanathan, V., Kumari, A., Singh, A., Kumar, A., Sharma, P., Chopra, S., Sharma, S., Raje, C.I., Singh, T.P.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Glyceraldehyde-3-phosphate dehydrogenase | A, B [auth C], C [auth B], D | 341 | Acinetobacter baumannii | Mutation(s): 0 Gene Names: epd, gap, A7M90_08740, Aba7835_13565, ABCAM1_1226, ABR2091_1144, APD17_19525, AYR68_05750, B7L45_13105, B9W25_17760... EC: 1.2.1 | 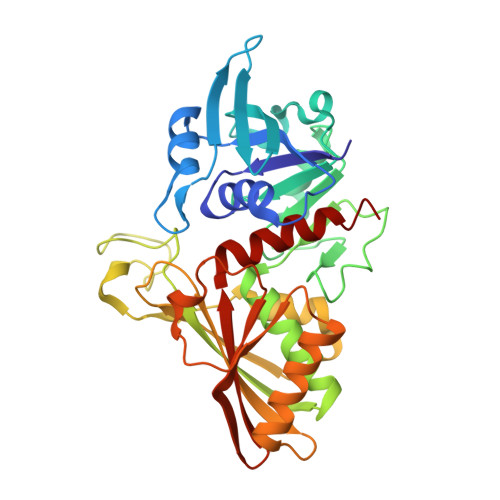 |
UniProt | |||||
Find proteins for A0A059ZTK9 (Acinetobacter baumannii) Explore A0A059ZTK9 Go to UniProtKB: A0A059ZTK9 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A0A059ZTK9 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| NAD (Subject of Investigation/LOI) Query on NAD | E [auth A], H [auth C], K [auth B], N [auth D] | NICOTINAMIDE-ADENINE-DINUCLEOTIDE C21 H27 N7 O14 P2 BAWFJGJZGIEFAR-NNYOXOHSSA-N |  | ||
| SO4 (Subject of Investigation/LOI) Query on SO4 | F [auth A] G [auth A] I [auth C] J [auth C] L [auth B] | SULFATE ION O4 S QAOWNCQODCNURD-UHFFFAOYSA-L |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 147.646 | α = 90 |
| b = 167.88 | β = 90 |
| c = 152.269 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| XDS | data reduction |
| XSCALE | data scaling |
| MOLREP | phasing |
| Coot | model building |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Science and Engineering Research Board (SERB) | India | -- |
| Indian Council of Medical Research | India | -- |