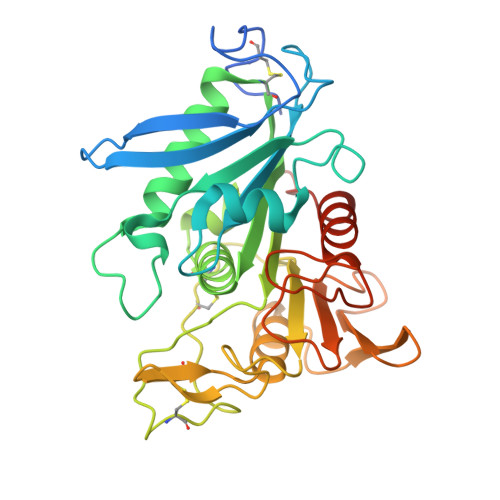
Identification and structural characterization of a novel acetyl xylan esterase from Aspergillus oryzae.
Yamada, C., Kato, T., Shiono, Y., Koseki, T., Fushinobu, S.(2025) FEBS J
- PubMed: 39876052
- DOI: https://doi.org/10.1111/febs.17420
- Primary Citation of Related Structures:
9J07, 9J08 - PubMed Abstract:
Acetyl xylan esterase plays a crucial role in the degradation of xylan, the major plant hemicellulose, by liberating acetic acid from the backbone polysaccharides. Acetyl xylan esterase B from Aspergillus oryzae, designated AoAxeB, was biochemically and structurally investigated. The AoAxeB-encoding gene with a native signal peptide was successfully expressed in Pichia pastoris as an active extracellular protein. The purified recombinant protein had pH and temperature optima of 8.0 and 30 °C, respectively, and was stable up to 35 °C. The optimal substrate for hydrolysis by purified recombinant AoAxeB among a panel of α-naphthyl esters was α-naphthyl acetate. Recombinant AoAxeB catalyzed the release of acetic acid from wheat arabinoxylan. The release of acetic acid from wheat arabinoxylan increased synergistically with xylanase addition. No activity was detected for the methyl esters of ferulic, p-coumaric, caffeic, or sinapic acids. The crystal structures of AoAxeB in the apo and succinate complexes were determined at resolutions of 1.75 and 1.90 Å, respectively. Although AoAxeB has been classified in the Esterase_phb family in the ESTerases and alpha/beta-Hydrolase Enzymes and Relatives (ESTHER) database, its structural features partly resemble those of ferulic acid esterase in the FaeC family. Phylogenetic analysis also indicated that AoAxeB is located between the clades of the two families. Docking analysis provided a plausible binding mode for xylotriose substrates acetylated at the 2- or 3-hydroxy position. This study expands the current knowledge of the structures of acetyl xylan esterases and ferulic acid esterases that are required for complete plant biomass degradation.
Organizational Affiliation:
School of Agriculture, Meiji University, Kawasaki, Japan.