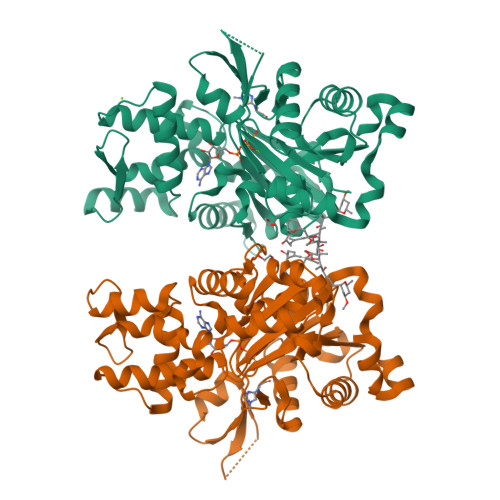
Structural basis of swinholide a binding to actin
Klenchin, V.A., King, R., Tanaka, J., Marriott, G., Rayment, I.(2005) Chem Biol 12: 287-291
- PubMed: 15797212
- DOI: https://doi.org/10.1016/j.chembiol.2005.02.011
- Primary Citation of Related Structures:
1YXQ - PubMed Abstract:
Marine toxins targeting the actin cytoskeleton represent a new and promising class of anti-cancer compounds. Here we present a 2.0 A resolution structure of swinholide A, a marine macrolide, bound to two actin molecules. The structure demonstrates that the actin dimer in the complex does not represent a physiologically relevant entity, for the two actin molecules do not interact with each other. The swinholide A actin binding site is the same as that targeted by toxins of the trisoxazole family and numerous actin binding proteins, highlighting the importance of this site in actin polymerization. The observed structure reveals the mechanism of action of swinholide A and provides a structural framework about which to design new agents directed at the cytoskeleton.
Organizational Affiliation:
Department of Biochemistry, University of Wisconsin at Madison, 433 Babcock Drive, Madison, Wisconsin 53706, USA.