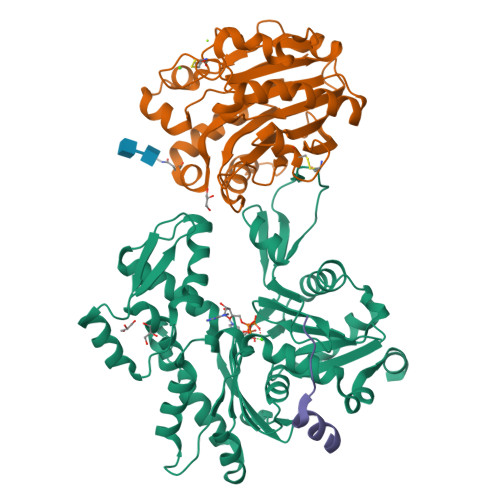
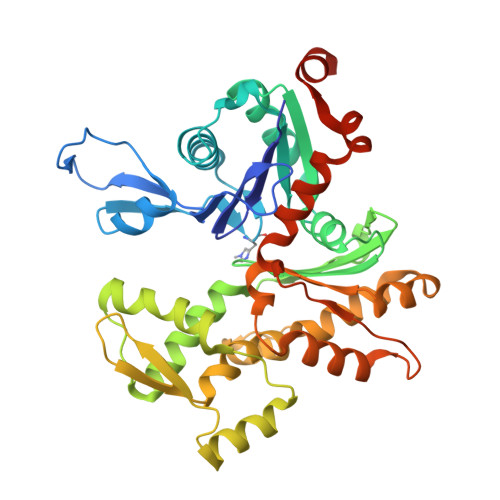
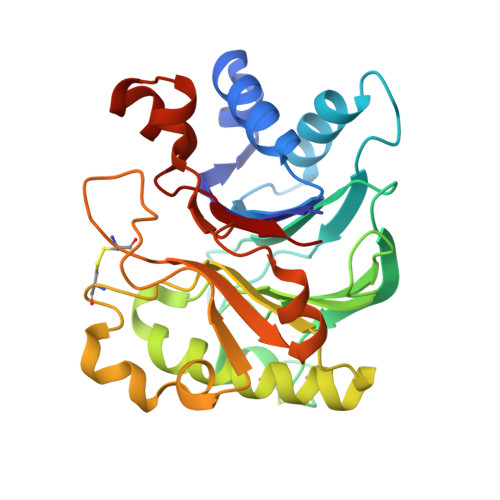
Actin-bound structures of Wiskott-Aldrich syndrome protein (WASP)-homology domain 2 and the implications for filament assembly
Chereau, D., Kerff, F., Graceffa, P., Grabarek, Z., Langsetmo, K., Dominguez, R.(2005) Proc Natl Acad Sci U S A 102: 16644-16649
- PubMed: 16275905
- DOI: https://doi.org/10.1073/pnas.0507021102
- Primary Citation of Related Structures:
2A3Z, 2A40, 2A41, 2A42 - PubMed Abstract:
Wiskott-Aldrich syndrome protein (WASP)-homology domain 2 (WH2) is a small and widespread actin-binding motif. In the WASP family, WH2 plays a role in filament nucleation by Arp2/3 complex. Here we describe the crystal structures of complexes of actin with the WH2 domains of WASP, WASP-family verprolin homologous protein, and WASP-interacting protein. Despite low sequence identity, WH2 shares structural similarity with the N-terminal portion of the actin monomer-sequestering thymosin beta domain (Tbeta). We show that both domains inhibit nucleotide exchange by targeting the cleft between actin subdomains 1 and 3, a common binding site for many unrelated actin-binding proteins. Importantly, WH2 is significantly shorter than Tbeta but binds actin with approximately 10-fold higher affinity. WH2 lacks a C-terminal extension that in Tbeta4 becomes involved in monomer sequestration by interfering with intersubunit contacts in F-actin. Owing to their shorter length, WH2 domains connected in tandem by short linkers can coexist with intersubunit contacts in F-actin and are proposed to function in filament nucleation by lining up actin subunits along a filament strand. The WH2-central region of WASP-family proteins is proposed to function in an analogous way by forming a special class of tandem repeats whose function is to line up actin and Arp2 during Arp2/3 nucleation. The structures also suggest a mechanism for how profilin-binding Pro-rich sequences positioned N-terminal to WH2 could feed actin monomers directly to WH2, thereby playing a role in filament elongation.
Organizational Affiliation:
Boston Biomedical Research Institute, Watertown, MA 02472, USA.