Structural and functional insights into the Asp1/2/3 complex mediated secretion of pneumococcal serine-rich repeat protein PsrP.
Guo, C., Feng, Z., Zuo, G., Jiang, Y.L., Zhou, C.Z., Chen, Y., Hou, W.T.(2020) Biochem Biophys Res Commun 524: 784-790
- PubMed: 32037091
- DOI: https://doi.org/10.1016/j.bbrc.2020.01.146
- Primary Citation of Related Structures:
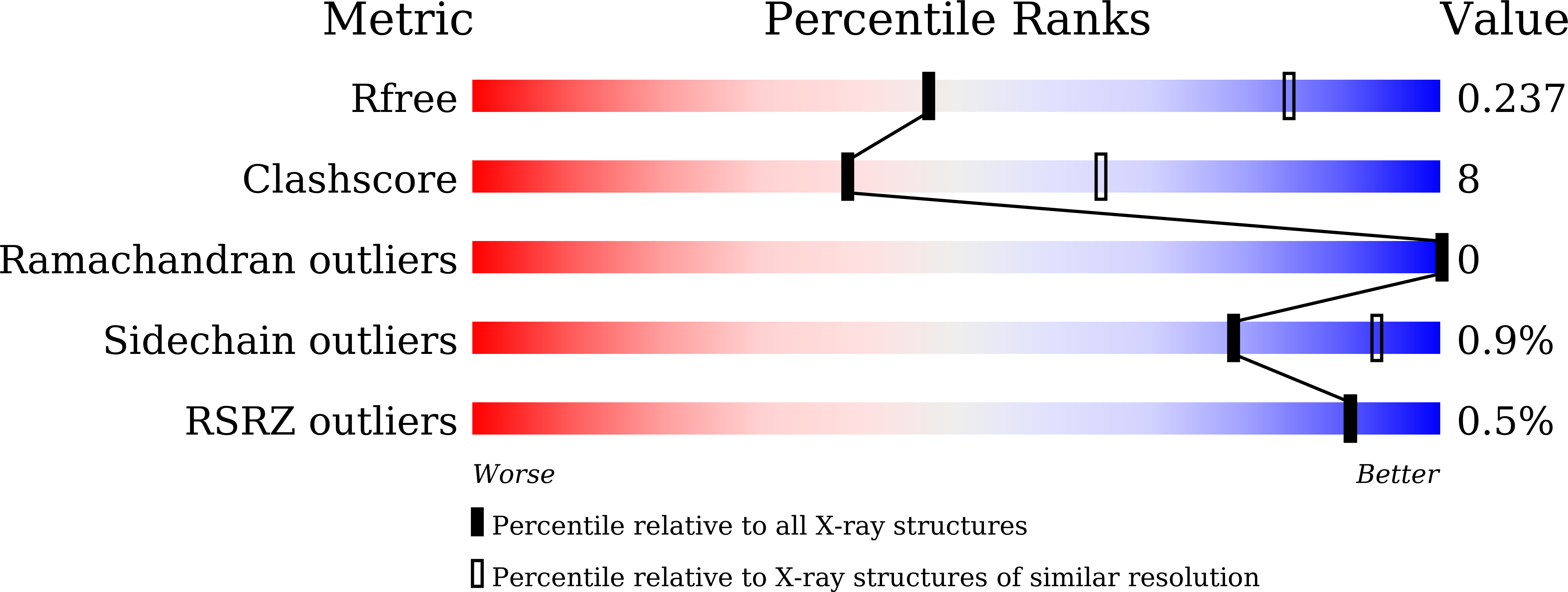
6LNW - PubMed Abstract:
The accessory sec system consisting of seven conserved components is commonly distributed among pathogenic Gram-positive bacteria for the secretion of serine-rich-repeat proteins (SRRPs). Asp1/2/3 protein complex in the system is responsible for both the O-acetylation of GlcNAc and delivering SRRPs to SecA2. However, the molecular mechanism of how Asp1/2/3 transport SRRPs remains unknown. Here, we report the complex structure of Asp1/2/3 from Streptococcus pneumoniae at 2.9 Å. Further functional assays indicated that Asp1/2/3 can stimulate the ATPase activity of SecA2. In addition, the deletion of asp1/2/3 gene resulted in the accumulation of a secreted version of PsrP with an altered glycoform in protoplast fraction of the mutant cell, which suggested the modification/transport coupling of the substrate. Altogether, these findings not only provide structural basis for further investigations on the transport process of SRRPs, but also uncover the indispensable role of Asp1/2/3 in the accessory sec system.
Organizational Affiliation:
Hefei National Laboratory for Physical Sciences at the Microscale and School of Life Sciences, University of Science and Technology of China, Hefei, Anhui, 230026, China.