A mutant of the nitrile hydratase from Geobacillus pallidus having enhanced thermostability
Van Wyk, J.C., Cowan, D.A., Danson, M.J., Tsekoa, T.L., Sayed, M.F., Sewell, B.T.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Nitrile hydratase | 207 | Aeribacillus pallidus | Mutation(s): 1 EC: 4.2.1.84 | 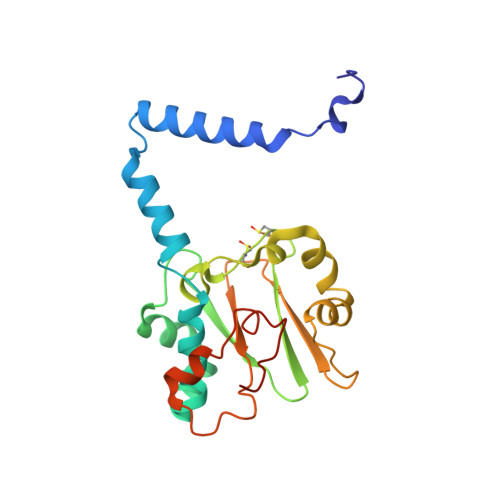 | |
UniProt | |||||
Find proteins for Q84FS5 (Bacillus sp. RAPc8) Explore Q84FS5 Go to UniProtKB: Q84FS5 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q84FS5 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Nitrile hydratase subunit beta | 229 | Aeribacillus pallidus | Mutation(s): 2 EC: 4.2.1.84 | 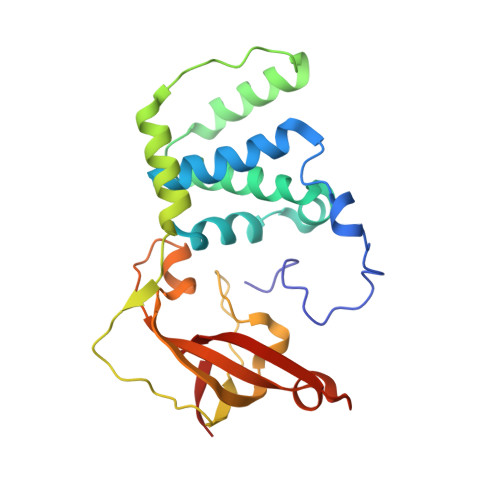 | |
UniProt | |||||
Find proteins for Q84FS6 (Bacillus sp. RAPc8) Explore Q84FS6 Go to UniProtKB: Q84FS6 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q84FS6 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| CO (Subject of Investigation/LOI) Query on CO | C [auth A] | COBALT (II) ION Co XLJKHNWPARRRJB-UHFFFAOYSA-N |  | ||
| CL (Subject of Investigation/LOI) Query on CL | D [auth A] | CHLORIDE ION Cl VEXZGXHMUGYJMC-UHFFFAOYSA-M |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| CSD Query on CSD | A | L-PEPTIDE LINKING | C3 H7 N O4 S |  | CYS |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 106.18 | α = 90 |
| b = 106.18 | β = 90 |
| c = 82.88 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| d*TREK | data scaling |
| PDB_EXTRACT | data extraction |
| d*TREK | data reduction |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Research Foundation in South Africa | United Kingdom | -- |
| Royal Society | United Kingdom | -- |