Selective targeting of oncogenic hotspot mutations of the HER2 extracellular domain
Bang, I., Hattori, T., Leloup, N., Corrado, A., Koide, A., Geles, K., Buck, E., Koide, S.To be published.
Experimental Data Snapshot
Starting Models: experimental, in silico
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Receptor tyrosine-protein kinase erbB-2/hIgG1 Fc domain fusion | 894 | Homo sapiens | Mutation(s): 1 Gene Names: ERBB2, HER2, MLN19, NEU, NGL EC: 2.7.10.1 | 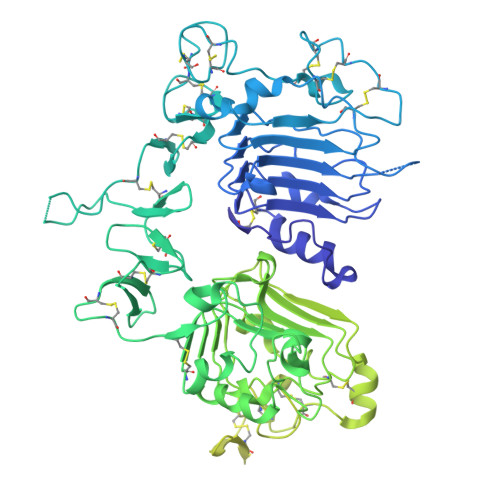 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P04626 (Homo sapiens) Explore P04626 Go to UniProtKB: P04626 | |||||
PHAROS: P04626 GTEx: ENSG00000141736 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P04626 | ||||
Glycosylation | |||||
| Glycosylation Sites: 1 | Go to GlyGen: P04626-1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Variable Heavy chain of TL1 Fab | B [auth C] | 257 | Homo sapiens | Mutation(s): 0 | 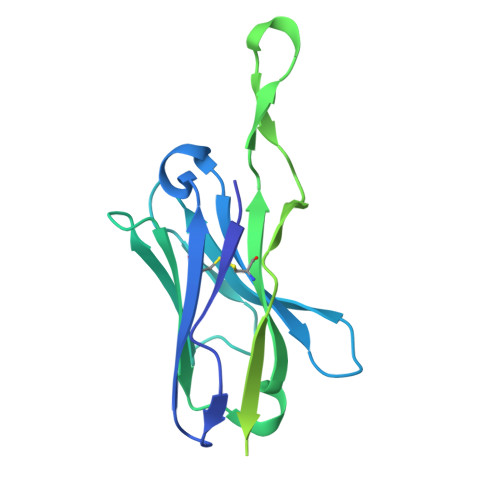 |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Variable Light chain of TL1 Fab | C [auth B] | 215 | Homo sapiens | Mutation(s): 0 | 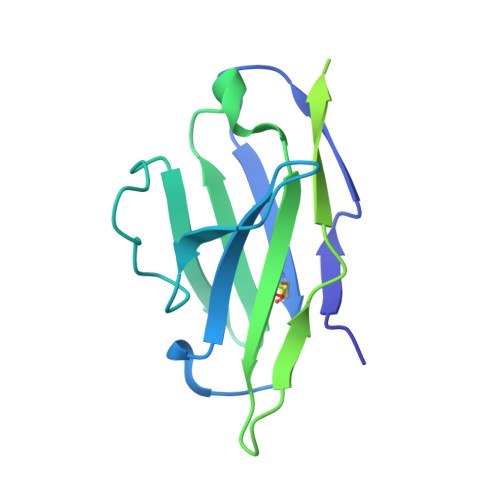 |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Task | Software Package | Version |
|---|---|---|
| MODEL REFINEMENT | PHENIX | 1.20.1_4487 |
| MODEL REFINEMENT | Coot | 0.9.8.1 |
| RECONSTRUCTION | cryoSPARC | 4.2.1 |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Not funded | -- |