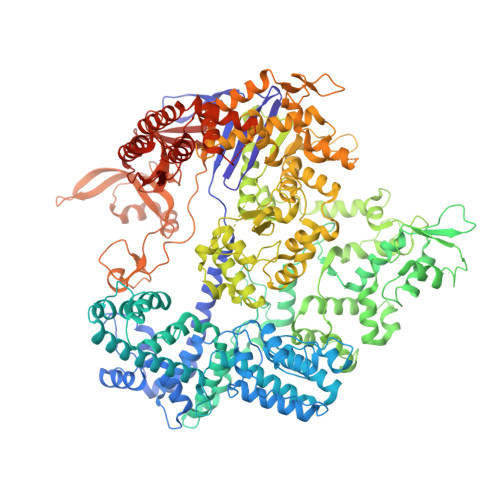
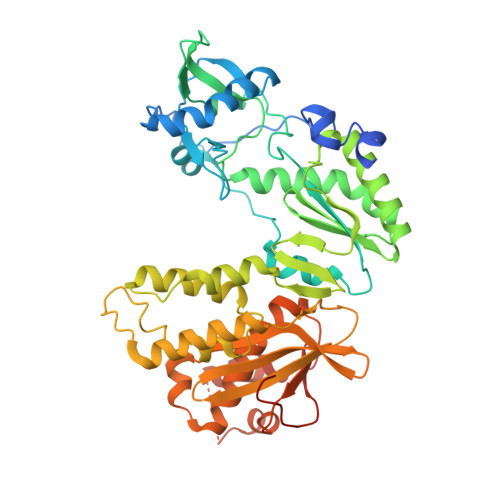
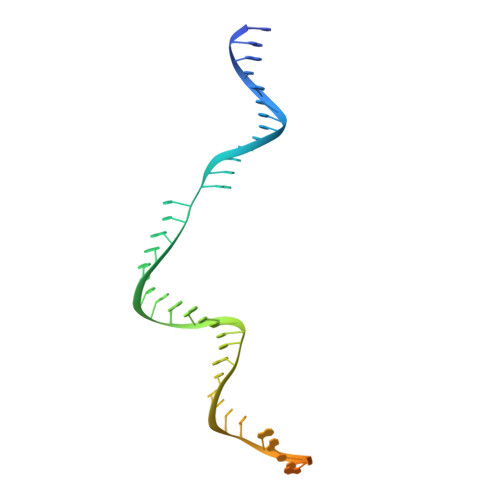
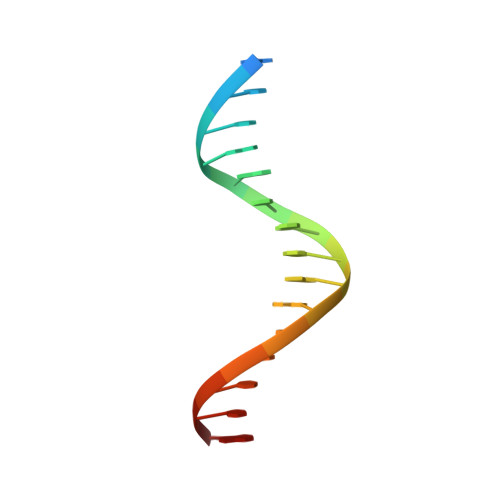
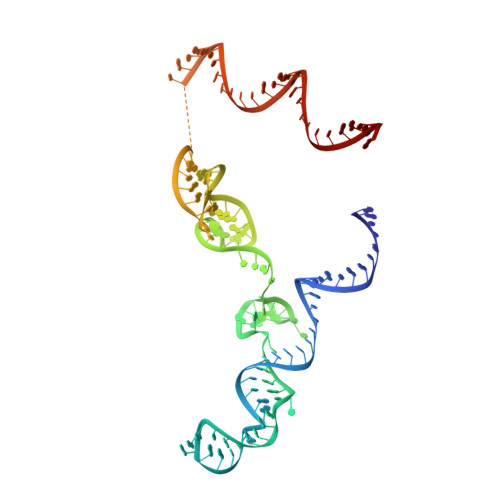
Structural basis for pegRNA-guided reverse transcription by a prime editor.
Shuto, Y., Nakagawa, R., Zhu, S., Hoki, M., Omura, S.N., Hirano, H., Itoh, Y., Zhang, F., Nureki, O.(2024) Nature 631: 224-231
- PubMed: 38811740
- DOI: https://doi.org/10.1038/s41586-024-07497-8
- Primary Citation of Related Structures:
8WUS, 8WUT, 8WUU, 8WUV, 8YGJ - PubMed Abstract:
The prime editor system composed of Streptococcus pyogenes Cas9 nickase (nSpCas9) and engineered Moloney murine leukaemia virus reverse transcriptase (M-MLV RT) collaborates with a prime editing guide RNA (pegRNA) to facilitate a wide variety of precise genome edits in living cells 1 . However, owing to a lack of structural information, the molecular mechanism of pegRNA-guided reverse transcription by the prime editor remains poorly understood. Here we present cryo-electron microscopy structures of the SpCas9-M-MLV RTΔRNaseH-pegRNA-target DNA complex in multiple states. The termination structure, along with our functional analysis, reveals that M-MLV RT extends reverse transcription beyond the expected site, resulting in scaffold-derived incorporations that cause undesired edits at the target loci. Furthermore, structural comparisons among the pre-initiation, initiation and elongation states show that M-MLV RT remains in a consistent position relative to SpCas9 during reverse transcription, whereas the pegRNA-synthesized DNA heteroduplex builds up along the surface of SpCas9. On the basis of our structural insights, we rationally engineered pegRNA variants and prime-editor variants in which M-MLV RT is fused within SpCas9. Collectively, our findings provide structural insights into the stepwise mechanism of prime editing, and will pave the way for the development of a versatile prime editing toolbox.
Organizational Affiliation:
Department of Biological Sciences, Graduate School of Science, The University of Tokyo, Tokyo, Japan.