A High-Resolution Crystallographic Study of Cytochrome c6: Structural Basis for Electron Transfer in Cyanobacterial Photosynthesis.
Zhang, B., Xu, Y., Liu, S., Chen, S., Zhao, W., Li, Z., Wang, J., Zhao, W., Zhang, H., Dong, Y., Gong, Y., Sheng, W., Cao, P.(2025) Int J Mol Sci 26
- PubMed: 39859539
- DOI: https://doi.org/10.3390/ijms26020824
- Primary Citation of Related Structures:
9KRC, 9KRD, 9KRR - PubMed Abstract:
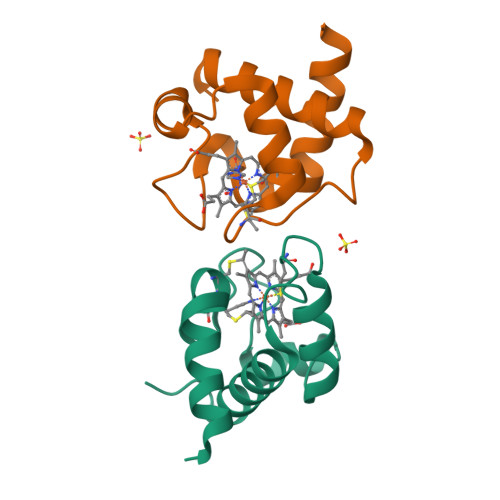
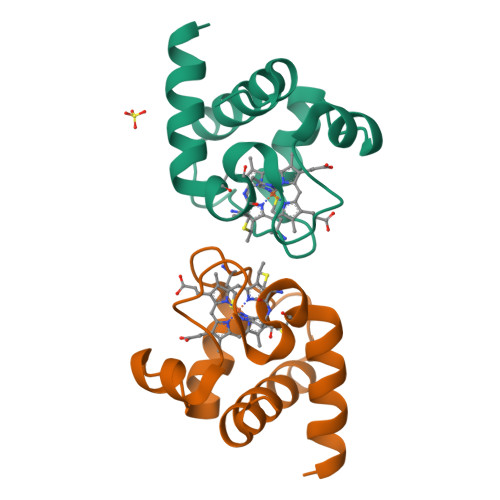
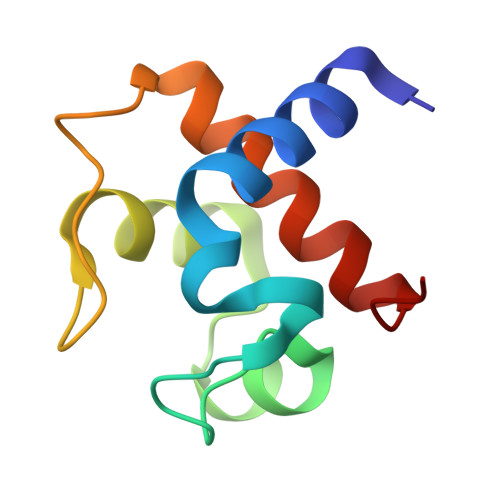
Cyanobacterial cytochrome c6 (Cyt c6) is crucial for electron transfer between the cytochrome b6f complex and photosystem I (PSI), playing a key role in photosynthesis and enhancing adaptation to extreme environments. This study investigates the high-resolution crystal structures of Cyt c6 from Synechococcus elongatus PCC 7942 and Synechocystis PCC 6803, focusing on its dimerization mechanisms and functional implications for photosynthesis. Cyt c6 was expressed in Escherichia coli using a dual-plasmid co-expression system and characterized in both oxidized and reduced states. X-ray crystallography revealed three distinct crystal forms, with asymmetric units containing 2, 4, or 12 molecules, all of which consist of repeating dimeric structures. Structural comparisons across species indicated that dimerization predominantly occurs through hydrophobic interactions within a conserved motif around the heme crevice, despite notable variations in dimer positioning. We propose that the dimerization of Cyt c6 enhances structural stability, optimizes electron transfer kinetics, and protects the protein from oxidative damage. Furthermore, we used AlphaFold3 to predict the structure of the PSI-Cyt c6 complex, revealing specific interactions that may facilitate efficient electron transfer. These findings provide new insights into the functional role of Cyt c6 dimerization and its contribution to improving cyanobacterial photosynthetic electron transport.
Organizational Affiliation:
College of Chemistry and Life Science, Beijing University of Technology, Beijing 100124, China.